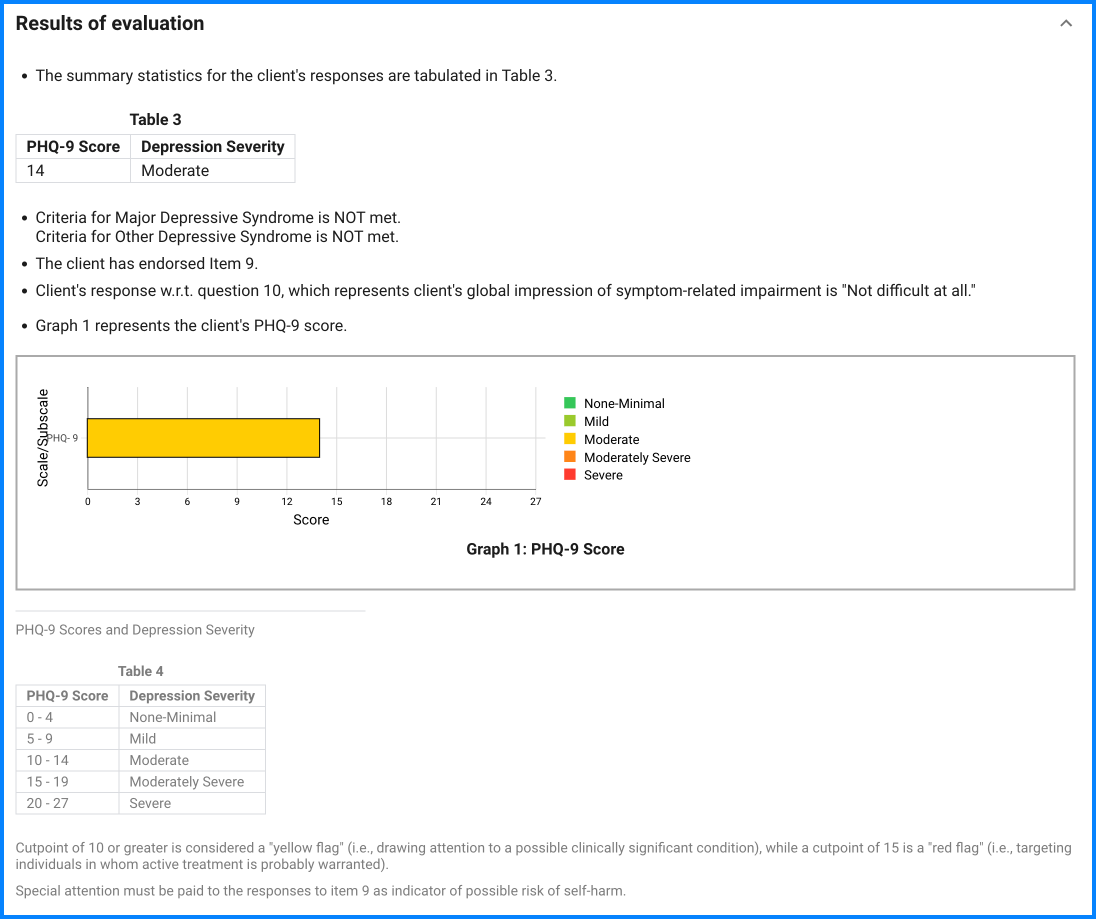
Since the questionnaire relies on client self-report, all responses should be verified by the clinician, and a definitive diagnosis is made on clinical grounds taking into account how well the client understood the questionnaire, as well as other relevant information from the client.
Diagnosis of Major Depressive Disorder or Other Depressive Disorder also require impairment of social, occupational, or other important areas of functioning (Question #10) and ruling out normal bereavement, a history of a Manic Episode (Bipolar Disorder), a physical disorder and medication, or other drug as the biological cause of the depressive symptoms.
Endorsement of item 9 (Suicidal Thoughts) is an indicator of possible suicidal risk. A final decision about the actual risk of self-harm requires further assessment and clinical interview. Further assessment of suicide risk can be made by asking about the “4 P’s”: past suicide attempts, a plan, probability of completing suicide, and preventive factors.
The final question on the PHQ-9 asks the clients to report ―how difficult have these problems made it for you to do your work, take care of things at home, or get along with other people? This single client-rated difficulty item is not used in calculating the PHQ-9 score or diagnosis but rather represents the patient‘s global impression of symptom-related impairment. It may be useful in decisions regarding initiation of or adjustments to treatment since it is strongly associated with both psychiatric symptom severity as well as multiple measures of impairment and health-related quality of life.
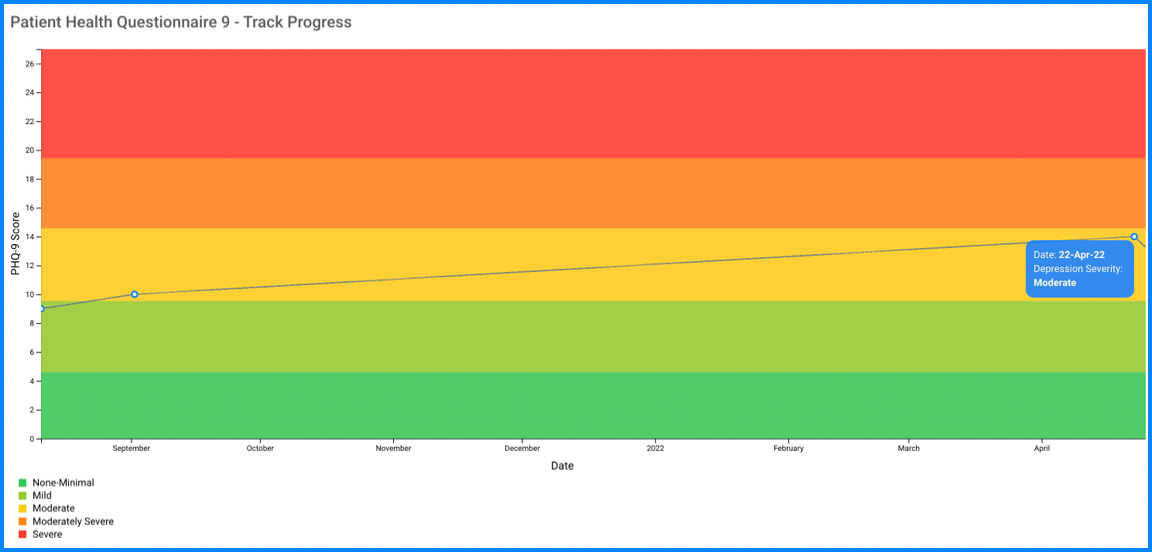
Sensitivity to change for monitoring treatment outcomes: A particularly important use of a measure is its responsiveness to changes of condition severity over time. This is well-established for the PHQ-9 which is increasingly used as a measure to assess the level of depression severity (for initial treatment decisions) as well as an outcome tool (to determine treatment response)
After making a provisional diagnosis with the PHQ-9, there are additional clinical considerations that may affect decisions about management and treatment.